BioDodo Research Digest #5: 2024 Biotech Honorable Mentions
Exploring the pivotal technological and scientific breakthroughs that defined 2024 in biotechnology.
Foreword
This post is a collaboration with
. Together we also wrote an in-depth analysis of the Top Five Biotech Breakthroughs of 2024, published on his SubStack page . These topics will not be covered here so make sure not to miss it!As we step into 2025, biotech enthusiasts will be facing the new year with an healthy dose of optimism. Over the past year, the biotech space has witnessed significant progress, marked by groundbreaking technological developments and the advancement of existing molecules into clinical trials.
In the Top Five Biotech Breakthroughs of 2024, we explored the breakthroughs that had the greatest impact—whether realized or with high potential—on the biotech market. Here, we shift our focus to technological leaps that, though less discussed, represent crucial progress and are poised to take center stage in the near future.
Without further ado, let’s begin our 2024 biotech honorable mentions list!
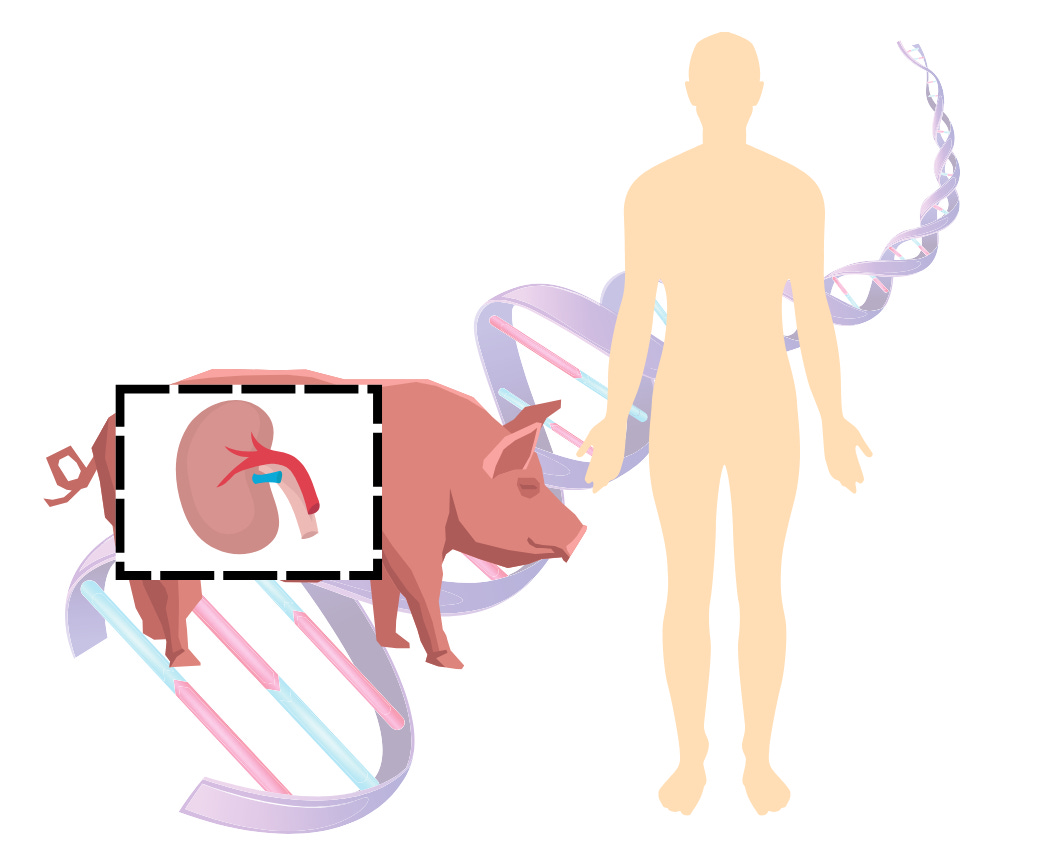
Advancements in xenotransplantation - First lab-grown kidneys transplant
In previous articles (link 1; link 2), we explored the use of genetically modified pigs to address the organ transplantation crisis, focusing on experimental procedures performed on brain-dead patients to assess the safety and feasibility of xenotransplantation. While those efforts were critical first steps, 2024 marked a significant milestone with three groundbreaking transplants of genetically modified pig kidneys into living human patients, further solidifying the potential of this technology to address the organ shortage crisis.
The first transplant of the year took place on March 16, 2024, when Richard Slayman, a 62-year-old patient with end-stage kidney disease, received a genetically modified pig kidney (MSG). The kidney, sourced from a pig with multiple genetic modifications to minimize rejection and viral transmission, functioned well post-transplant. Although Mr. Slayman passed away two months later due to unrelated causes, the success of the procedure demonstrated the feasibility of xenotransplantation in living patients.
A second transplant was performed on April 15, 2024, for Lisa Pisano, who also showed promising early recovery (Wired). However, complications arising from an unrelated mechanical heart pump led to the removal of the organ after 47 days. Despite these challenges, her case provided valuable insights into the integration of xenotransplanted organs in complex medical scenarios.
The most recent case occurred in November 2024, when Towana Looney, a 53-year-old woman, received a pig kidney at NYU Langone Health (UT). Looney’s transplant may have marked a turning point, as she experienced immediate improvements in energy levels and was discharged from the hospital in early December with the kidney still functioning well. Her case has sparked optimism that xenotransplantation could soon transition from experimental use to routine clinical practice.
These groundbreaking transplants represent significant progress, but challenges remain, including long-term safety, immune compatibility, and regulatory approval. United Therapeutics, through its subsidiary Revivicor, leads the development of the genetically modified pig organs used in these trials. Companies like Qihan Biotech and eGenesis are advancing gene-editing technologies to enhance future organ models. Together, these efforts are paving the way for larger-scale clinical trials and potential commercialization.
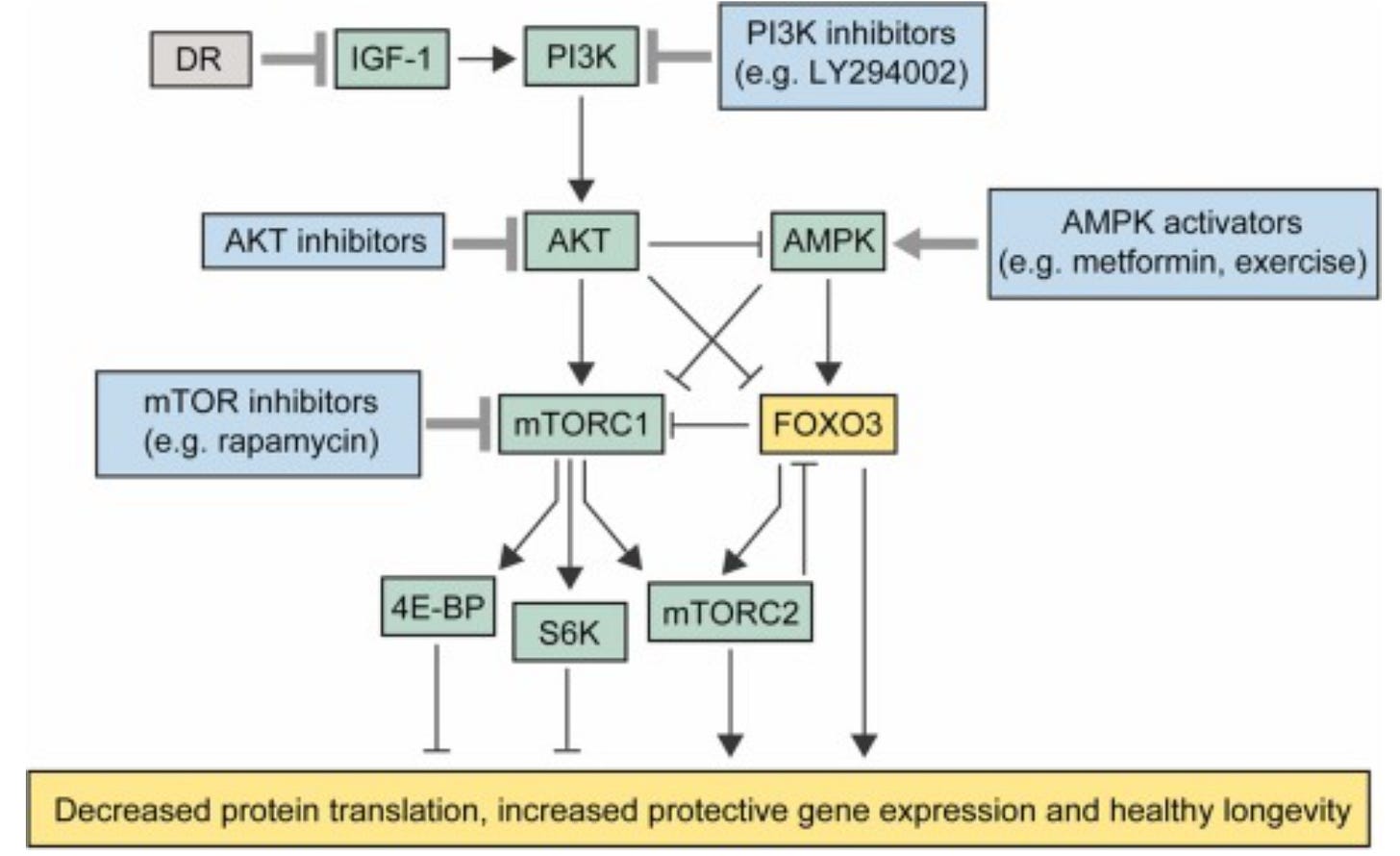
Targeting mTOR/FOXO3 to combat ageing
Ageing is a multifaceted biological phenomenon that takes place at the biochemical, genetic, epigenetic, cellular and systemic levels. Reducing the negative effects of advancing age and increasing human healthy life-span (healthspan) is a holy grail of biotechnology that continues to draw attention due to its enormous potential for revolutionizing healthcare. In recent years, the metabolic aspects of aging have gained substantial traction, and findings from basic research are now starting to make their way into clinical settings.
Metformin is normally used in treating type-2 diabetes. By stimulating the activation of AMP-activated protein kinase (AMPK), metformin causes the inhibition of the mTORC1 complex via activation of the transcription factor FOXO3. Downregulation of this pathway has been shown to significantly increase the lifespan of c. elegans and mice in preclinical studies.
In 2024, a randomized phase-2 clinical trial was carried out to evaluate the safety and efficacy of metformin in slowing geographic atrophy (GA) progression, an age-associated eye disease that affects >5 million people worldwide. Another study that marked 2024 focused on the mTOR inhibitor rapamycin, which has also been shown to promote longevity in mice but for which safety and efficacy effects in humans are limited. Human subjects in the rapamycin group showed improved bone mineral density and muscle mass. Additionally, women also reported improved self-reported physical pain. In both studies, even though the effects on a broader range of longevity-associated metrics is still missing, the treatments were found to be safe in healthy adults, paving the way for future clinical research in this area.
Single cell foundation models
AI has undeniably played a central role in shaping this year’s biotech landscape. Although protein structure prediction has rightfully received a lot of attention (See Top 5 biotech breakthroughs that shaped 2024), there is more to it. This year, two single cell foundation models were released, representing a huge step forward for computational biology.
In brief, single cell foundation models are capable of learning gene expression patterns from cells across different tissues, disease and treatment conditions (Eisenstein et al., 2024; Lotfollahi et al., 2024). Similar to how GPT-4 and DALL-E model text and images, single-cell foundation models can be used to simulate the activity of individual cells. In other words, these models bring us very close to creating digital twins of cells.
The analogy between single cell foundation models and LLMs is not limited to an abstract level. The same technology that enabled GPT-4 was used to build the single cell foundation models scGPT and scFoundation. Rather than on text, like GPT-4, these models were pre-trained using the transcriptional profiles of tens of millions of cells to learn application-agnostic knowledge on individual cells. The models can subsequently be fine-tuned to carry out a variety of specialized tasks, including gene expression and cell type prediction.
So far, single-cell foundation models are designed to handle transcriptomics data, and they have already demonstrated exceptional performance on tasks for which specialized tools are available. The integration of data collected from different modalities, such as chromatin accessibility, proteomics and metabolomics, holds the promise to be the next frontier in single-cell foundation models.
mRNA editing
CRISPR/Cas has revolutionized the way a lot of diseases are treated by allowing to “fix” defective genes. However, off-target effects when editing DNA are virtually irreversible, and thus come with a huge potential risk. Given the transient nature of messenger RNA (mRNA), RNA editing technologies represent an equally effective strategy to cure diseases while avoiding the risk of irreversible unwanted changes to the genome.
Two promising technologies to edit RNA with therapeutic purposes are Adenosine deaminase acting on RNA (ADAR) and exon editing by trans-splicing. The first one is used to replace the base adenine with another molecule, inosine, thus allowing to edit mRNA one base ad the time. This mechanism has been exploited to create a novel treatment for alpha-1 antitrypsin deficiency (AATD), which has been tested for safety and efficacy in a Phase-1 clinical trial that ended at the end of 2024.
Exon editing by trans-splicing exploits a phenomenon that naturally occurs in eukaryotic cells to replace entire exons in pre-mRNA. In brief, when a gene is transcribed to mRNA it contains “unused” nucleotides sequences called introns. During splicing, introns are removed and exons, the “useful” sequences, are rejoined together. When two exons from the same original mRNA molecule are joined, the process is known as cis-splicing. In trans-splicing exons that are spliced together come from different mRNA molecules, including those that are deliberately introduced in cells. In 2024, a clinical trial for a treatment based on this technology was approved and is currently ongoing, aimed to tackle the Stargardt disease, which causes progressive loss of vision.
Cell therapies for autoimmune diseases
The past year saw a surge in the number of cell therapies for autoimmune diseases reaching clinical studies. Several chimeric antigen receptor T cells (CAR-T) products against systemic lupus erythematosus, multiple sclerosis and myasthenia gravis have been tested in compassionate use programs as well as phase 1 and phase 2 clinical trials. The results are promising: CAR-T are emerging as a viable alternative to monoclonal antibodies, a de facto standard in the treatment of autoimmune disease. Further, CAR-T used in this therapeutic context appear safer than they are when used as treatment for cancer, where they have raised concern due to side effects such as cytokines storm and neurotoxicity.
New technologies in this space are also being tested in the preclinical stage. Chimeric auto-antibody receptors (CAAR) are designed to make CAR-T cells even more precise, targeting only cells that express antibodies against a given antigen targeted by an autoimmune disease. CAR natural killer cells (CAR-NK) against lupus nephritis are also under development and expected to reach clinical studies soon, as well as allogeneic CAR-T, making off-the-shelf cell therapies an ever closer reality.
Activin receptor blocker for improved body composition
Weight loss therapeutics have taken the biotech industry by storm, particularly GLP-1 receptor agonists, which are featured at the top of our Top 5 Biotech Breakthroughs of 2024. Despite their effectiveness in achieving significant weight loss, these drugs often lead to a significant loss of muscle mass, which can have detrimental effects, particularly for older adults. This has sparked a surge of interest in improving the quality of weight loss by preserving muscle mass during fat reduction. Activin receptor inhibitors are emerging as a promising solution to achieve this balance.
Activin receptor inhibitors work by blocking activin type II receptors (ActRIIA and ActRIIB), which are part of the myostatin signaling pathway involved in muscle and fat regulation. This blockade prevents activins and myostatin, proteins that inhibit muscle growth, from binding to their receptors. The result is a dual effect: reduction of fat mass and promotion of muscle hypertrophy (Nunn et al., 2024). Unlike traditional weight loss drugs, activin receptor inhibitors do not rely solely on reducing calorie intake or increasing energy expenditure. Instead, they target the underlying biological mechanisms that regulate muscle and fat composition, offering a more sophisticated approach to metabolic health.
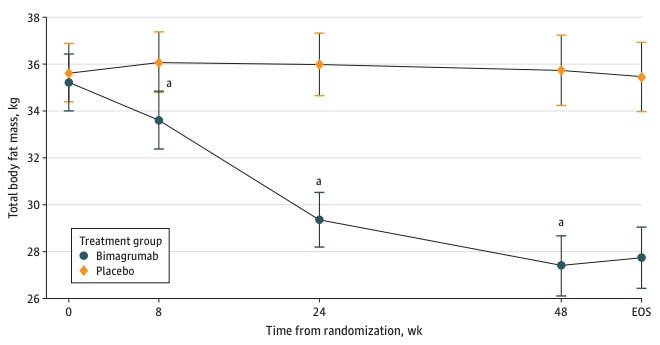
Clinically, the field is gaining traction, with several activin receptor inhibitors in various stages of development. Preclinical models have shown that activin blockade paired with GLP-1 drugs preserves skeletal muscle mass and enhances fat loss (Nunn et al., 2024). A clinical study of an activin inhibitor as a monotherapy has shown promise in reducing fat mass while preserving lean muscle in a Phase II study (Heymsfield et al., 2021). Eli Lilly’s acquisition of Versanis Bio, the owner of this activin blocker, reflects confidence in this technology’s potential, but these drugs are still in the late-stage clinical pipeline. Regulatory approval and widespread availability are likely a few years away. However, the growing body of evidence suggests that activin receptor inhibitors could soon become a cornerstone of next-generation obesity treatments, addressing critical gaps in current therapies and reshaping the landscape of weight loss therapeutics.
Non-hormonal contraceptives
There has been a growing demand for non-hormonal contraception options as traditional hormonal methods, which rely on altering the body’s endocrine system, face increasing scrutiny for their short-term and long-term side effects. Non-hormonal contraceptives offer an opportunity to create safer, effective, side-effect-free birth control. These methods function by targeting specific mechanisms involved in fertilization or sperm viability, offering the potential for highly targeted, reversible, and on-demand contraception.
One of the most promising developments came from Evofem Biosciences, which advanced its novel vaginal gel, Phexxi, designed to regulate vaginal pH and create an inhospitable environment for sperm. The gel, already approved as a contraceptive, saw renewed interest in 2024 as ongoing studies explored its potential to double as a preventative for sexually transmitted infections (STIs), further increasing its utility.
In parallel, research into male non-hormonal contraception strategies is also gaining momentum. One leading approach focuses on disrupting sperm development by inhibiting retinoic acid receptor alpha (RARα) (Shi et al., 2025). Preclinical studies showed that YCT-529, a compound from YourChoice Therapeutics, was 99% effective and completely reversible with no side effects. Early-phase clinical trials of this compound have yielded promising results, raising hopes for its eventual approval.
Other male non-hormonal contraceptives are emerging in preclinical studies. A notable breakthrough showed the ability to immobilize sperm temporarily, providing effective contraception for several hours without impacting sperm production or long-term fertility (Ballbach et al., 2023). Another strategy targets proteins involved in sperm motility, effectively preventing sperm from reaching the egg (Gomes et al., 2023). These advancements represent a monumental shift in contraceptive options, offering men effective, reversible, and side-effect-free solutions.
Non-hormonal contraceptives are still in their early stages of clinical development, but the demand for alternatives to hormonal methods is driving rapid innovation. These options have the potential to expand the reproductive health, giving individuals more choices tailored to their unique physiological and lifestyle needs. As these technologies mature, they could transform contraception by prioritizing safety, reversibility, and accessibility.
Bacteriophage Therapies as a next generation antibiotic
As the global crisis of antibiotic resistance escalates, bacteriophage therapies are emerging as a promising alternative to traditional antibiotics. Bacteriophages, or phages, are viruses that specifically infect and kill bacteria. Unlike broad-spectrum antibiotics, phages can be engineered to target specific bacterial strains, leaving the beneficial microbiota intact. This precision makes phage therapy a highly attractive solution for combating multi-drug-resistant infections.
Phage therapies leverage the natural ability of bacteriophages to kill bacteria. They can be used as phage cocktails, which combine multiple phages to target diverse bacterial strains or prevent resistance. Advances in genetic engineering have further expanded their functionality. For example, synthetic phages can now carry unique antimicrobial payloads, such as biofilm-degrading enzymes to penetrate bacterial biofilms or CRISPR-based antimicrobials to disrupt bacterial genomes. These innovations enable phages to go beyond bacterial lysis, addressing some of the limitations of traditional antibiotics.
Currently, most phage therapies are used under emergency Investigational New Drug (IND) applications, allowing compassionate use for patients with life-threatening infections. There are no commercialized phage therapies yet, but several companies are on the cusp of achieving regulatory approval.
Locus Biosciences is leading the charge with its LBP-EC01 therapy for treating recurrent urinary track infections (UTIs). The company completed Phase 2 Part I of the ELIMINATE trial in 2023, an open-label study that met both primary and secondary outcomes. Results, published in The Lancet Infectious Diseases, demonstrated strong efficacy and safety, showing high bacterial clearance rates in patients with E. coli UTIs (Kim et al., 2024).
The trial has now progressed to Phase 2 Part II, a double-blind study designed to confirm these findings in a larger patient population. If successful, LBP-EC01 will become the first commercialized bacteriophage product, a landmark achievement in the field of antimicrobial therapies.
Beyond UTIs, phage therapies are being developed for a range of other infections, including respiratory infections in cystic fibrosis, prosthetic joint infections, and chronic wound infections. Companies like Armata Pharmaceuticals are exploring similar innovations, broadening the scope of phage therapy to address critical gaps in treating drug-resistant infections.
The success of Locus Biosciences and its peers could redefine how we approach bacterial infections, transforming bacteriophages from a niche experimental tool into a cornerstone of precision medicine.
If you're curious about the top 5 breakthroughs that shaped the biotech landscape in 2024, check out the rest of the post on Neural NeXus!
Concluding remarks
2024 was a good year for biotech. We are looking ahead with excitement, which is shared by an ever growing community here on SubStack. More people every day express their interest in these topics, because let’s be honest life sciences and biotech are cool as hell. If you are interested and willing to support this community there are few things you can do. Subscribing to the newsletter and liking the post have a huge impact in helping others finding us.
Sharing with your friends is also a great way to make this community grow.
Which innovation do you think had the most impact already? Which one the most potential?
References
Advancements in Xenotransplantation
In a First, Genetically Edited Pig Kidney Is Transplanted Into Human
First pig kidney transplant in a person: what it means for the future
Wang, Y.; Chen, G.; Pan, D.; Guo, H.; Jiang, H.; Wang, J.; Feng, H.; He, S.; Du, J.; Zhang, M.; Li, T.; Wang, Y.; Yu, H.; Gan, H.; Wen, Q.; Song, Z.; Li, D.; Yu, Y.; Wang, H.; Li, B.; You, Y.; Zhou, S.; Wang, M.; Liu, L.; Xu, L.; Yang, M.; Pei, H.; Zhang, K.; Chen, Z. K. Pig-to-Human Kidney Xenotransplants Using Genetically Modified Minipigs. Cell Rep Med 2024, 5 (10), 101744.
mTOR/FOXO3 as Targets to Address Aging
McIntyre, R. L.; Liu, Y. J.; Hu, M.; Morris, B. J.; Willcox, B. J.; Donlon, T. A.; Houtkooper, R. H.; Janssens, G. E. Pharmaceutical and Nutraceutical Activation of FOXO3 for Healthy Longevity. Ageing Research Reviews 2022, 78, 101621.
Zhang, Z.; Yang, R.; Zi, Z.; Liu, B. A New Clinical Age of Aging Research. Trends in Endocrinology & Metabolism 2024, S1043276024002236.
Harinath, G.; Lee, V.; Nyquist, A.; Moel, M.; Morgan, S. L.; Isman, A.; Zalzala, S. Safety and Efficacy of Rapamycin on Healthspan Metrics after One Year: PEARL Trial Results. medRxiv September 24, 2024, p 2024.08.21.24312372.
Shen, L. L.; Keenan, J. D.; Chahal, N.; Taha, A. T.; Saroya, J.; Ma, C. J.; Sun, M.; Yang, D.; Psaras, C.; Callander, J.; Flaxel, C.; Fawzi, A. A.; Schlesinger, T. K.; Wong, R. W.; Bryan Leung, L.-S.; Eaton, A. M.; Steinle, N. C.; Telander, D. G.; Afshar, A. R.; Neuwelt, M. D.; Lim, J. I.; Yiu, G. C.; Stewart, J. M. METformin for the MINimization of Geographic Atrophy Progression (METforMIN): A Randomized Trial. Ophthalmology Science 2024, 4 (3), 100440.
Single-Cell Foundation Models
Eisenstein, M. Foundation Models Build on ChatGPT Tech to Learn the Fundamental Language of Biology. Nature Biotechnology 2024, 42 (9), 1323–1325.
Lotfollahi, M.Toward Learning a Foundational Representation of Cells and Genes. Nat Methods 2024, 21 (8), 1416–1417.
Cui, H.; Wang, C.; Maan, H.; Pang, K.; Luo, F.; Duan, N.; Wang, B. scGPT: Toward Building a Foundation Model for Single-Cell Multi-Omics Using Generative AI. Nat Methods 2024, 21 (8), 1470–1480.
Hao, M.; Gong, J.; Zeng, X.; Liu, C.; Guo, Y.; Cheng, X.; Wang, T.; Ma, J.; Zhang, X.; Song, L. Large-Scale Foundation Model on Single-Cell Transcriptomics. Nat Methods 2024, 1–11.
mRNA Editing
Doi, A.; Delaney, C.; Tanner, D.; Burkhart, K.; Bell, R. D. RNA Exon Editing: Splicing the Way to Treat Human Diseases. Molecular Therapy - Nucleic Acids 2024, 35 (3), 102311.
Lenharo, M. Move over, CRISPR: RNA-Editing Therapies Pick up Steam. Nature 2024, 626 (8001), 933–934
Cell Therapies for Autoimmune Diseases
Harrison, C. CAR-Ts Sweep into Autoimmunity. Nature Biotechnology 2024, 42 (7), 995–997.
Activin Receptor Blockers for Improved Body Composition
Chuong, V., Farokhnia, M., Khom, S., Pince, C.L., Elvig, S.K., Vlkolinsky, R., Marchette, R.C., Koob, G.F., Roberto, M., Vendruscolo, L.F. and Leggio, L., 2023. The glucagon-like peptide-1 (GLP-1) analogue semaglutide reduces alcohol drinking and modulates central GABA neurotransmission. JCI insight, 8(12).
Heymsfield, S.B., Coleman, L.A., Miller, R., Rooks, D.S., Laurent, D., Petricoul, O., Praestgaard, J., Swan, T., Wade, T., Perry, R.G. and Goodpaster, B.H., 2021. Effect of bimagrumab vs placebo on body fat mass among adults with type 2 diabetes and obesity: a phase 2 randomized clinical trial. JAMA network open, 4(1), pp.e2033457-e2033457.
Nunn, E., Jaiswal, N., Gavin, M., Uehara, K., Stefkovich, M., Drareni, K., Calhoun, R., Lee, M., Holman, C.D., Baur, J.A. and Seale, P., 2024. Antibody blockade of activin type II receptors preserves skeletal muscle mass and enhances fat loss during GLP-1 receptor agonism. Molecular Metabolism, 80, p.101880.
Rodriguez, P.J., Cartwright, B.M.G., Gratzl, S., Brar, R., Baker, C., Gluckman, T.J. and Stucky, N.L., 2024. Semaglutide vs tirzepatide for weight loss in adults with overweight or obesity. JAMA Internal Medicine.
Non-Hormonal Contraceptives
Balbach, M., Rossetti, T., Ferreira, J., Ghanem, L., Ritagliati, C., Myers, R.W., Huggins, D.J., Steegborn, C., Miranda, I.C., Meinke, P.T. and Buck, J., 2023. On-demand male contraception via acute inhibition of soluble adenylyl cyclase. Nature communications, 14(1), p.637.
Gomes, A.A., Santos, N.C., Rosa, L.R., Borges, R.J., Fontes, M.R., Hamil, K.G., O’Rand, M.G. and Silva, E.J., 2023. Interactions of the male contraceptive target EPPIN with semenogelin-1 and small organic ligands. Scientific reports, 13(1), p.14382.
Shi, R., Wolgemuth, D.J. and Georg, G.I., 2025. Development of the retinoic acid receptor alpha-specific antagonist YCT-529 for male contraception: A brief review. Contraception, p.110809.
Wang, W., Volkow, N.D., Wang, Q., Berger, N.A., Davis, P.B., Kaelber, D.C. and Xu, R., 2024. Semaglutide and Opioid Overdose Risk in Patients With Type 2 Diabetes and Opioid Use Disorder. JAMA Network Open, 7(9), pp.e2435247-e2435247.